Calculation of partition functions and thermodynamic potentials for diatomic molecules
Main results
- Currently, tasks related to the calculation of partition functions are very relevant. The methods used today are based on the calculation of partition functions using spectroscopic constants;
- The most widely used approach is based on the Born-Oppenheimer approximation, which allows one to devide contributions of electronic, vibrational, and rotating degrees of freedom to the total internal partition function. In this case, polynamial approximations for energy states of atoms and molecules are used;
- It is worth noting that at low and medium temperatures, the energies of vibrational-rotational states are quite accurately expressed analytically for most diatomic molecules. At high temperatures, high vibrational and rotational levels are highly populated, and such a method does not give a good estimate for the vibrational-rotational energies of such states;
- At high temperatures, more accurate results when calculating the partition functions and thermodynamic properties can be obtained by calculating the second virial coefficient for two interacting atoms, and then calculating the statistical and thermodynamic properties. In addition, concomitant calculation problems are considered, such as obtaining exact interaction potentials of atoms in a molecule.
Results of numerical simulation
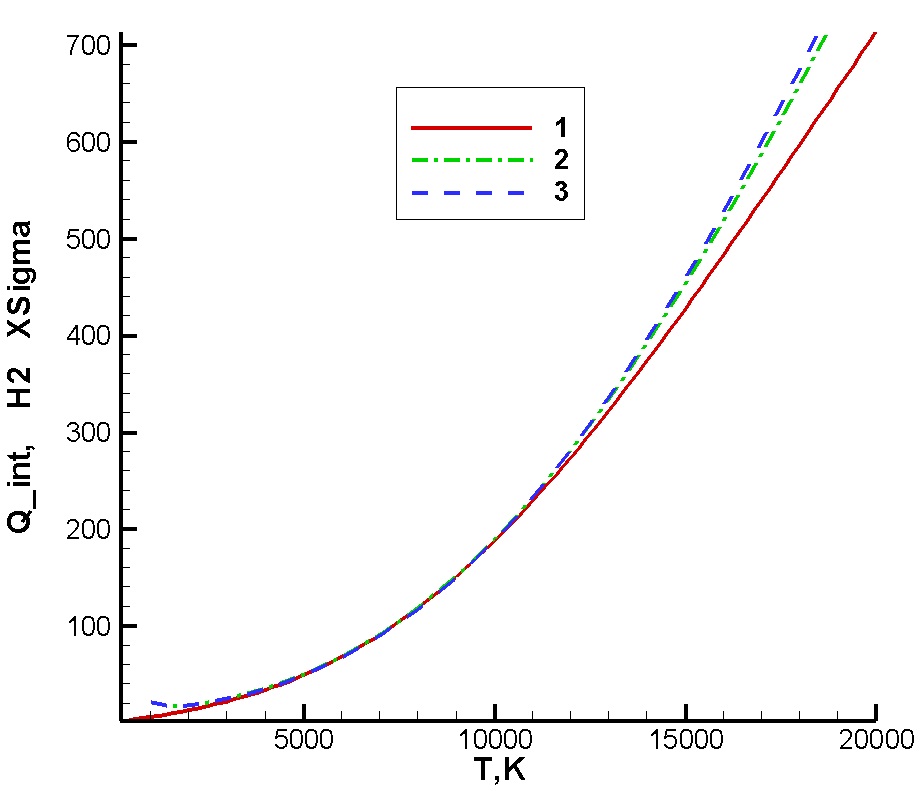 |
Internal partition function calculated for molecular hydrogen in ground electronic state: 1 - result obtained using spectroscopic constants; 2 - virial coefficient, ab-initio potential; 3 - virial coefficient, Rydberg potential
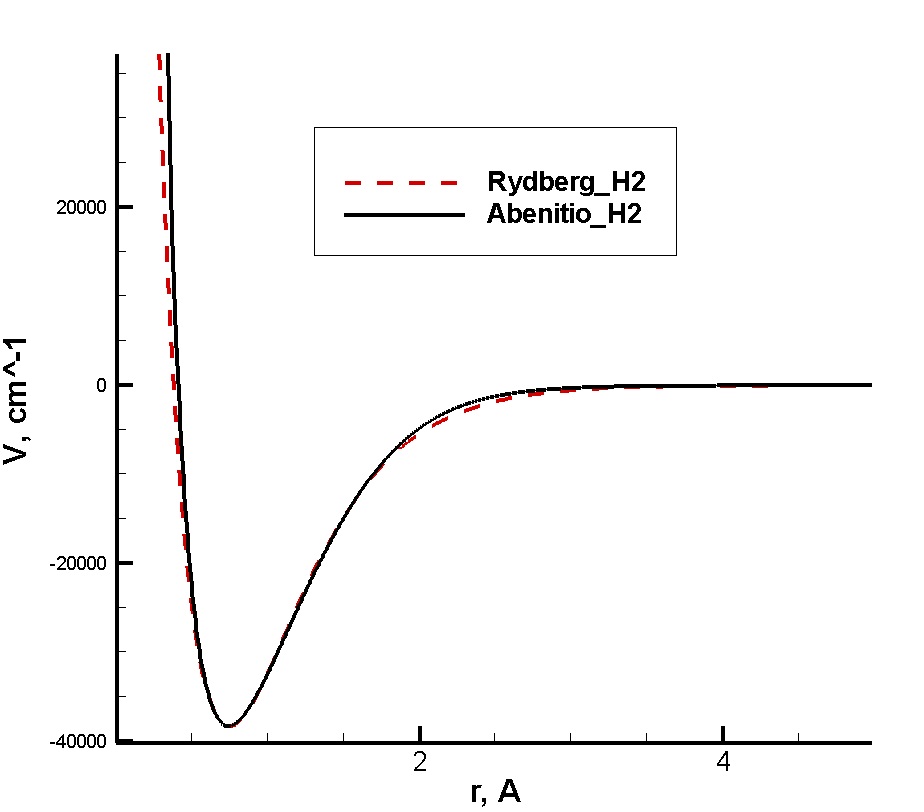 |
Potential curves for the ground electronic state of a hydrogen molecule. Even such minor differences in potential curves can significantly affect the final result
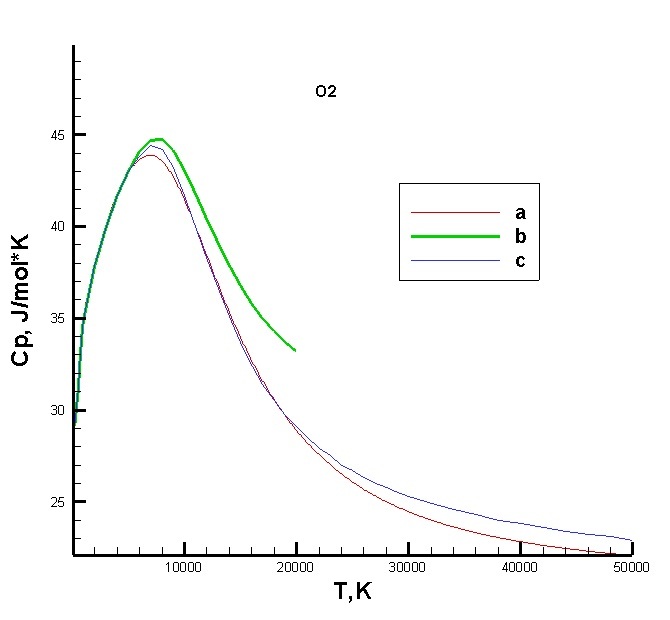 |
Calculation of specific heat for an oxygen molecule: a - the results are obtained on the basis of the constructed numerical model; b - the results are obtained from the tables of the JIHT RAS; c - the results are obtained from ESA tables STR-236. Statistical sums are used to determine various thermodynamic characteristics and optical properties of the medium

